Latest news
Scientific events
RMeS in the spotlight
À Nantes, Baptiste Charbonnier met au point des biomatériaux capables de traiter les pertes osseuses de la mâchoire et, ainsi, permettre la pose d’implants dentaires. Pour ce projet, le chercheur Inserm a obtenu un financement Atip-Avenir.

Lire l'article
Echosciences Pays de la Loire
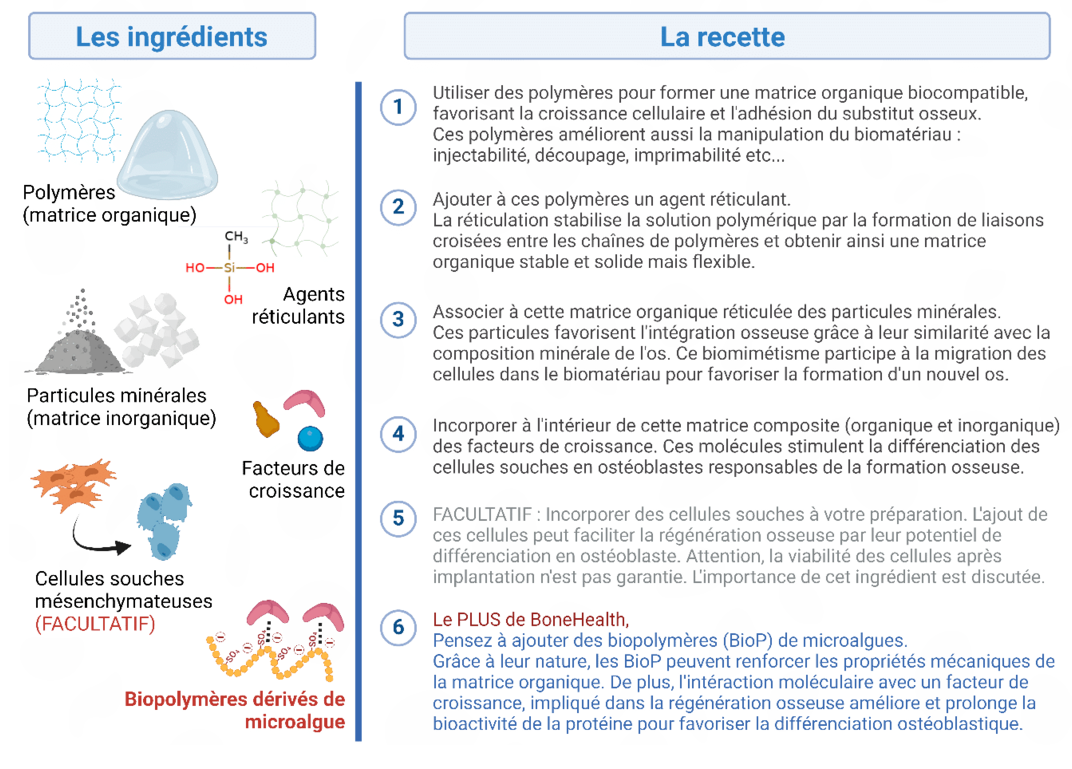
Article d'Arnaud Dulom "Intégration d'ingrédients dérivés de microalgues dans la formulation de biomatériaux en régénération osseuse"
https://www.echosciences-paysdelaloire.fr/articles/les-elements-essentiels-pour-formuler-des-biomateriaux-en-regeneration-osseuse
Le Figaro Santé
Entretien avec le PR Jérôme Guicheux "des cellules souches pour régénérer le cartilage"
Et si l’on pouvait un jour remplacer les cartilages pour le grand bien des articulations ?
Biomatériaux et transplantations de cellules ouvrent des perspectives.


 Projet AMI ingénierie & santé DECISION
Projet AMI ingénierie & santé DECISIONmoDèle mathEmatique prédiCtif de matériaux de régénératIon oSseuse antIbactériens pOur le traitement de la péri-implaNtite.
Résumé : Les infections péri-implantaires sont une complication très répandue en raison de l’exposition permanente aux germes infectieux. De plus, la péri-implantite (PI) a été qualifiée de pathologie majeure en raison de l’absence de protocole de traitement standardisé. La principale raison est une décontamination insuffisante de la surface de l’implant. Dans ce contexte, les matériaux composites injectables se présentent comme une approche clinique intéressante. Ce concept repose sur l’utilisation de biomatériaux composites ostéoconducteurs associés à des molécules antibactériennes (généralement des antibiotiques). Compte tenu de la préoccupation mondiale de la résistance aux antimicrobiens, nous avons émis l’hypothèse du remplacement de l’antibiotique par des polymères antibactériens. Nous développerons un matériau composite antibactérien (MCA) avec : l’acide hyaluronique silanisé comme matrice polymérique et le phosphate de calcium biphasique comme matrice ostéoconductrice avec différentes quantités de biopolymères antimicrobiens. Dans ce projet, notre objectif est de mettre au point un traitement médical personnalisé. Pour y parvenir, l’originalité de notre projet consiste à développer un modèle mathématique capable d’approcher le comportement de nos MCA dans leur environnement afin de prédire leur efficacité. In fine, l’objectif est de pouvoir associer ce modèle avec les données cliniques des patients afin de prédire quel MCA devra être utilisé selon la situation clinique du patient.
Porteur : RMeS : Gildas RETHORE
Partenaires : GEM : (Central) Gregory Legrain, Baptiste Girault
IMN : Stéphane Cuenot

The REJOINT team is pleased to announce the funding of the "Novel strategies for Osteoarthritis treatment based on innate LYMPhoid Cells modulation (OLYMPiC2024)" ANR/DFG PRCI project, coordinated by Marie-Astrid Boutet (StratOA group, RMeS) and Irene Mattiola (Institute of Microbiology, Infectious Diseases and Immunology, Charité Universitätsmedizin, Berlin, Germany).
The OLYMPiC2024 project aims to:
- Decipher the diversity and spatial distribution of innate lymphoid cells (ILC) in joint tissues at different stages of OA and the correlation with the clinical features of the patients.
- Investigate the contribution of ILC subsets to the pathophysiology of OA and evaluating therapeutic agents aimed at modulating ILC activation in OA models.
- Study the role of the microbiota-dependent regulation of ILC in relevant models of OA, to bring the proof of concept for a diet-based microbiota/ILC-modulating strategy in OA dog patients.
 TARMac Project
TARMac ProjectThe REJOINT team is delighted to announce the funding of the TARMac project in the framework of the “Equipe FRM 2023” (Medical Research Foundation Team 2023). The FRM Team-TARMac will be coordinated by Jerome Guicheux with Marie-Astrid Boutet as a co-coordinator.
Involving more than 15 collaborators from the Rejoint team, TARMac will also benefit from the expertise of the Pitzalis’ Lab (London, UK), Escriou’s lab (Paris, FR) as well as the Preclinical Research and Investigation Center (CRIP) at the Oniris Vet school (Nantes, FR).
Our team will receive a 3-years funding starting in 2024 and aiming at better understanding macrophages diversity in the synovial tissue to help accurately stratify osteoarthritis (OA) patients, and design novel and efficient RNA-based treatments with a personalized approach. TARMac is organized into 3 work packages (WP) as follow:
- WP1. Characterise the synovial MΦ heterogeneity and its association with the disease phenotype and severity to develop a stratification for OA patients.
- WP2. Validate an innovative drug delivery strategy that allows the sustained release of therapeutic RNAs to efficiently modulate MΦ pathogenesis in OA joints.
- WP3. Bring the proof of concept that modulating the activity of specific MΦ subsets in murine models of OA efficiently prevents disease progression and bring the proof of safety in dogs.
This project, which involves multiple technologies and covers a broad range of our team members’ expertise along with a strong collaborative network, possesses all the ingredients for advancing scientific research in OA with a huge potential to accelerate the translation of basic scientific observations into patients’ benefits.
 PEPR CARN
PEPR CARN
As part of France 2030's acceleration strategies, one action is dedicated to the funding of translational research through the Priority Research Programs and Equipment (PEPR). The REJOINT team is delighted to announce a PEPR funding within the Biotherapies and Bioproduction of Innovative Therapies (BBiT) program. UMR 7057-MSC (F. Gazeau) and UMR 1229-RMeS (J. Guicheux) will co-lead the CARN project (3.5 M€) dedicated to the development of biotherapies based on the local delivery of therapeutic RNA by functionalized hybrid extracellular vesicles (EVs) for joint diseases. This project will also involve the UMR 8612 (E. Fattal), UMR 5253 (M. Morille), UMS 55-ART ARNm (C. Pichon) and UMR 1183-IRMB (C. Jorgensen).
CARN is a 4 year-project with a kick-off official date August 31, 2023.
It is organized into 5 workpackages (WP) as follows :
-WP1. Identification and validation of therapeutic RNAs for knee and intervertebral disc osteoarthritis. Leaders Claire Vinatier & Danièle Noel
-WP2. High yield production of mesenchymal stromal cells-derived EVs and RNAs and per-production hybridization of EVs and RNA. Leaders: Chantal Pichon & Florence Gazeau
-WP3. Engineering, optimization and functionalization of hybrid EVs. Leaders: Elias Fattal & Marie Morille
-WP4. Efficacy evaluation of hybrid EVs in vitro and in the zebrafish model. Leaders: Farida Djouad & Jerome Guicheux
-WP5. Pre-clinical studies in small (mice) and large (sheep) animal models of knee and disc osteoarthritis. Leaders: Catherine Le Visage & Christian Jorgensen
By proposing functionalized hybrid EVs decorated with recognition elements and loaded with disease-modifying therapeutic RNAs, CARN will open new therapeutic opportunities for hybrid EVs in the treatment of chronic inflammatory joint diseases.

Fondation d’entreprise Grand Ouest – Prix encouragement recherche – Projet ANDROID2
The REGOS team of the INSERM U1229-RMeS laboratory is pleased to announce the recent award to Guillaume MABILLEAU (INSERM U1229, RMeS) from the Fondation d’entreprise Grand Ouest. This award will help to pursue the development of dual GIP/GLP-2 analogues for the prevention and treatment of bone fragility.

Fondation Arthritis - SPOTT project
The REJOINT team is pleased to announce the funding of the "SPatial multi-Omic profiling of synovial tissue to uncover novel Therapeutic Targets in osteoarthritis (SPOTT)" project, coordinated by Marie-Astrid Boutet (StratOA group).
The SPOTT project aims to:
(i) Analyse the spatial multi-omic profile of OA synovial tissue to refine patients’ stratification,
(ii) Validate the expression of identified cellular or molecular targets for the future development of innovative therapeutic strategies.
SPOTT is an emergent project, which implements for the first-time breakthrough conceptual and technological approaches and will facilitate the development of larger impactful projects in the context of OA and beyond with a huge potential to accelerate the translation of basic scientific observations into patients’ benefits.
 ANR PVNS
ANR PVNS
Pigmented villonodular synovitis (PVNS) is a rare joint disease characterized by a benign proliferative lesion arising from synovia that results in a secondary inflammatory joint response, pain, and destruction. The PVNS project, coordinated by Benoit Le Goff and Frédéric Blanchard (RMeS, T-Syno group) in collaboration with Fabienne Coury (INSERM UMR1033, Lyon) and Jérôme Avouac (INSERM U1016 Paris), has 3 main aims:
- To better understand PVNS pathophysiology using high throughput technologies in a cohort of PVNS patients and identify new therapeutic targets
- To set up an in vitro platform to better understand the crosstalk between cells and test new drugs and therapeutic targets on cells and tissues from PVNS patients
- To use and further develop original mouse models of PVNS to test drugs or new therapeutic targets, combined with a local delivery system, for treating PVNS patients
 Funding of a new project by the Fondation de l'Avenir
Funding of a new project by the Fondation de l'Avenir
The objective of this project, coordinated by Benoit Le Goff and Frédéric Blanchard (RMeS, T-Syno group), is to provide proof of principle that the local delivery of molecules interacting with the CSF1 pathway is feasible and effective in in vitro and in vivo models of Pigmented villonodular synovitis (PVNS). We will test a panel of therapeutic molecules on PVNS cells and explants in vitro and measure their impact on cell viability. The active molecules will then be studied in a mouse model of synovial tissue xenograft from patients with PVNS.
 TARGET-OA
TARGET-OA
The REJOINT team of the INSERM U1229-RMeS laboratory is pleased to announce the recent funding of the “TARGET-OA: targeting the chondrocyte-derived CXCL12/endothelial cell CXCR4 axis in osteoarthritis” collaborative research project by the 2023 ANR Call. The ARGET-OA project is coordinated by Xavier Houard (INSERM UMRs 938 Centre de Recherche Saint Antoine, Paris) in collaboration with Marie-Hélène Lafage-Proust (INSERM U1059 SAINBIOSE, St Etienne) and Claire Vinatier (INSERM U1229-RMeS, AGE-OA group). In the TARGET-OA project, we propose that the CXCL12 / CXCR4 axis has a crucial role in the dialogue between hypertrophic chondrocytes and endothelial cells in OA. Our objectives are to define the role of this axis in pathological communication between chondrocytes and endothelial cells and to demonstrate that its targeting has a clinical interest in the treatment of OA.
 ANR DISPHPE
ANR DISPHPE
The REJOINT team of the INSERM U1229-RMeS laboratory is pleased to announce the recent funding of the DISPHPE collaborative research project by the 2022 ANR Call on Translational Research in Health. In the DISPHPE project: “Comprehensive study of DISP1 gene variants to reach genotype-phenotype relationships of congenital anterior midline defects” coordinated by Valérie Dupé (IGDR, Rennes), Anne Camus (BIODIV Group, INSERM U1229, RMeS) will develop human induced pluripotent stem cells-based experimental models to investigate key molecular and functional aspects of developmental disorders arising during embryogenesis and related to the signaling role of the notochord.

Dans le cadre de sa subvention aux projets individuels de recherche, la Société Française de Rhumatologie (SFR) a attribué une bourse [de 20000€] au projet « MoSCITO » porté par Romain GUIHO (La MOdulation de la Sénescence Cellulaire comme Innovation Thérapeutique dans la discOpathie dégénérative). En plus de faire progresser considérablement la caractérisation des cellules sénescentes dans le disque intervertébral, le projet MoSCITO a l'ambition de valider une nouvelle approche thérapeutique, ouvrant la voie à des progrès concrets et considérables dans la gestion clinique de la maladie discale.

- set up a multicentric biocollection of patients with PVNS;
- explore the mechanisms leading to the development of synovitis (inflammation, genetic mutations, vascular compartment) using high-throughput technologies
- evaluate in vitro the biological activity of several drugs, in particular those targeting the CSF1 pathway, on tissues /cells from PVNS patients.
 Bourse novartis DREAMER
Bourse novartis DREAMER
Dr Christelle DARRIEUTORT-LAFFITESujet de recherche : Etude de la cicatrisation d’un défect tendineux du supra-épineux chez la souris : analyse transcriptomique des populations cellulaires impliquées
Description du projet :
Résumé : Bien que les tendinopathies représentent une problématique médicale fréquente, les options thérapeutiques restent peu nombreuses. Le tendon ayant une faible vascularisation et une faible cellularité, ses capacités naturelles de régénération/réparation sont faibles. Ce contexte, associé à nos connaissances limitées de la biologie des cellules du tendon et de la régulation de la ténogenèse, font du traitement des tendinopathies un réel challenge médical et scientifique. Des données récentes issues du Single-cell RNA-Sequencing démontrent la présence de populations de ténocytes hétérogènes au sein du tendon sain. Aucune donnée n’est disponible sur les changements au sein de ces populations cellulaires après une lésion tendineuse.
Objectifs : Etudier par Single-Cell RNA-Sequencing les populations cellulaires présentes au sein du tendon lors des différentes phases de la cicatrisation après une lésion partielle induite chirurgicalement au niveau du supra-épineux. Les échantillons seront recueillis à différents temps : en phase inflammatoire (J7), à la phase de prolifération (J21) et lors de la phase de remodelage (J42). Les échantillons seront également étudiés en histologie et immunohistochimie aux différents temps expérimentaux.

ANR NEMESIS
Low back pain (LBP) has been associated with intervertebral disc (IVD) degeneration. The recent identification of the microRNA (miR) role in IVD biology and degeneration allows considering miRs as new targets, but also as new potential therapeutic agents. The development of in vivo delivery systems, particularly miR-loaded nanocarriers, has thus been an area of active research. As a whole, the objectives of the NEMESIS project are (i) to set-up the formulation and characterization of miR-loaded nanocarriers, (ii) to implement potency assays and assess the interactions of miR-loaded nanocarriers with target cells in vitro and in an ex vivo whole IVD culture system and (iii) to establish the proof of concept of miR-loaded nanocarriers as a new therapeutic option in the management of DDD in an ovine preclinical model and in canine clinical trials. The NEMESIS project is based on an inter- and multi-disciplinary consortium composed of J. Clouet (scientific leader, RMeS lab), M. Fusellier (scientific co-leader, RMeS lab and ONIRIS) and Elise Lepeltier (scientific partner, MINT lab, Angers).ATIP-Avenir SAMBA
Mandibular atrophy induced by the lack of a proper dentition is a major medical and socio-economic burden worldwide: the remaining bone volume is not 
MiRbone By SFR

 ANR-DYNAM-OA
ANR-DYNAM-OA
Osteoarthritis (OA) has no cure to date. To identify new therapeutic targets and screen drugs, researchers need in vitro OA joint models that are biologically relevant and reproducible. Bioprinting-assisted OA modeling is a promising avenue of research, yet bioprinting tools are limited. With the DYNAM-OA project, Vianney Delplace (BIOMAX group) propose to develop a new ultra-modular bioprinting toolbox, including a universal bioink platform and a strategy to produce gradient constructs, and apply these tools for the design of the first generation of bioprinted OA joint models.
 ANR-COBIOSTEM
ANR-COBIOSTEM
Traumatic brain injuries (TBI) are a critical public health and socio-economic problem throughout the world, with limited treatment options available. Cell transplantation offers a viable treatment strategy for patients with TBI, but transplanted neurons alone often rapidly die and fail to integrate brain tissue, leading to limited recovery. In the COBIOSTEM project, led by Afsaneh Gaillard (INSERM U1084 - LNEC), Vianney Delplace (BIOMAX group) will design a new generation of injectable hydrogels to improve the survival rate of transplanted cortical neurons, and enhance the long-term efficacy of cell transplantation to the brain.
 International Research Project (IRP) Inserm (2022) GLYCODISC
International Research Project (IRP) Inserm (2022) GLYCODISC
The goal of the GLYCODISC projet is to consolidate a cooperating relationship between France (PI: Catherine LE VISAGE, Inserm) and Hong Kong (PI: Barbara CHAN, The University of Hong Kong (HKU)), in the field of regenerative medicine for intervertebral disc disease and low back pain.
The funding (75 000 euros, duration 5 years) aims to primarily contribute to mobility and organization of meetings. The ultimate outcome of the program is to lay the foundation for a large-scale international collaborative project with scientists from both countries.
 ANR SmartIEs (2022)
ANR SmartIEs (2022)
The REJOINT team of the INSERM U1229-RMeS laboratory is pleased to announce the recent funding of the SmartIEs proposal as an ANR collaborative research project.
SmartIEs will be coordinated by Sylvia COLLIEC-JOUAULT (IFREMER) and will be carried out in collaboration with Stéphane CUENOT (Institut des Matériaux) and Catherine Le Visage (INSERM U1229, RMeS). The SmartIEs project will explore a cell-free strategy through conception of a smart hydrogel scaffold highly efficient to recruit regenerative progenitor cells and to stimulate their differentiation into appropriate cell lineages able to regenerate both cartilage and subchondral bone.
 CT scan-based morphometric comparison of human and canine lumbar spine generates instrumental data for intervertebral disc percutaneous surgery
CT scan-based morphometric comparison of human and canine lumbar spine generates instrumental data for intervertebral disc percutaneous surgeryN. Gavira, C. Decante, N. Bouhsina, D. Rouleau, B. Miannay, N. Bronsard, A. David, R. Jossier, O. Gauthier, A. Hamel, J. Guicheux, J. Clouet, M. Fusellier
Osteoarthritis and Cartilage Open
Abstract and More
 Comprehensive Characterization of Tissue Mineralization in an Ex Vivo Model
Comprehensive Characterization of Tissue Mineralization in an Ex Vivo ModelConstance Lesage, Pierre Guihard, Claire De Fourmestraux, Olivier Gauthier, Pierre Weiss, Jérome Guicheux, Aleksandra Mieczkowska, Joelle Véziers, Baptiste Charbonnier, Marie-Astrid Boutet, Guillaume Mabilleau
J Vis Exp
Abstract and More
 Unveiling the macrophage dynamics in osteoarthritic joints: From inflammation to therapeutic strategies
Unveiling the macrophage dynamics in osteoarthritic joints: From inflammation to therapeutic strategiesNicolas Gaigeard, Anaïs Cardon, Benoit Le Goff, Jérôme Guicheux, Marie-Astrid Boutet
Drug Discov Today
Abstract and more
 Boronate Ester Hydrogels for Biomedical Applications: Challenges and Opportunities
Boronate Ester Hydrogels for Biomedical Applications: Challenges and OpportunitiesLéa Terriac, Jean-Jacques Helesbeux, Yves Maugars, Jérôme Guicheux, Mark W. Tibbitt, and Vianney Delplace
https://pubs.acs.org/doi/10.1021/acs.chemmater.4c00507
Abstract and more
 Gut hormones and bone homeostasis: potential therapeutic implications
Gut hormones and bone homeostasis: potential therapeutic implicationsBéatrice Bouvard, Guillaume Mabilleau
https://www.nature.com/articles/s41574-024-01000-z
Abstract and more
 Gene therapies for osteoarthritis: progress and prospects
Gene therapies for osteoarthritis: progress and prospectsAnais Defois, Nina Bon, Mathieu Mével, David Deniaud, Yves Maugars, Jérôme Guicheux, Oumeya Adjali, Claire Vinatier
https://doi.org/10.1016/j.jcjp.2024.100186
Abstract and more
 Women's contribution to stem cell research for osteoarthritis: an opinion paper
Women's contribution to stem cell research for osteoarthritis: an opinion paperÉmilie Velot, Elizabeth R Balmayor, Lélia Bertoni, Susan Chubinskaya, Flavia Cicuttini , Laura de Girolamo, Magali Demoor, Brunella Grigolo, Elena Jones, Elizaveta Kon, Gina Lisignoli, Mary Murphy, Danièle Noël, Claire Vinatier, Gerjo J V M van Osch, Magali Cucchiarini
10.3389/fcell.2023.1209047
Abstract and more
 Automatic grading of intervertebral disc degeneration in lumbar dog spines
Automatic grading of intervertebral disc degeneration in lumbar dog spinesFrank Niemeyer, Fabio Galbusera, Martijn Beukers, René Jonas, Youping Tao, Marion Fusellier, Marianna A. Tryfonidou,
Cornelia Neidlinger-Wilke, Annette Kienle, Hans-Joachim Wilke
http://dx.doi.org/10.1002/jsp2.1326
Abstract and more
 Optimizing the physical properties of collagen/hyaluronan hydrogels by inhibition of polyionic complexes formation at pH close to the collagen isoelectric point
Optimizing the physical properties of collagen/hyaluronan hydrogels by inhibition of polyionic complexes formation at pH close to the collagen isoelectric pointStéphanie De Oliveira, Gregor Miklosic, Joëlle Veziers, Sébastien Grastilleur, Thibaud Coradin, Catherine Le Visage, Jérôme Guicheux, Matteo D'Este, Christophe Hélary
PMID: 37971365 DOI: 10.1039/d3sm01330h
Abstract and more

Development of a DNA damage-induced senescence model in osteoarthritic chondrocytes
Mélina Georget, Anaïs Defois, Romain Guiho, Nina Bon, Sophie Allain, Cécile Boyer, Boris Halgand, Denis Waast, Gaël Grimandi, Alban Fouasson-Chailloux, Jérôme Guicheux, Claire Vinatier
PMID: 33227437 PMCID: PMC7925350 DOI: 10.1016/j.joca.2020.11.004
Abstract and more
 Recommendations for intervertebral disc notochordal cell investigation: From isolation to characterization
Recommendations for intervertebral disc notochordal cell investigation: From isolation to characterizationWilliams R.J., Laagland L.T., Bach F.C., Ward L., Chan W., Tam V., Medzikovic A., Basatvat S., Paillat L., Vedrenne N.,
Snuggs J.W., Poramba-Liyanage D.W., Hoyland J.A., Chan D., Camus A., Richardson S.M., Tryfonidou M.A., Le Maitre C.L. (2023). Recommendations for intervertebral disc notochordal cell investigation: From isolation to characterization. JOR Spine 1272:1-22. DOI: 10.1002/jsp2.1272.
Abstract and more
 Osteoarthritic chondrocytes undergo a glycolysis-related metabolic switch upon exposure to IL-1b or TNF
Osteoarthritic chondrocytes undergo a glycolysis-related metabolic switch upon exposure to IL-1b or TNFAnais Defois , Nina Bon, Alexandre Charpentier , Melina Georget, Nicolas Gaigeard, Frederic Blanchard, Antoine Hamel, Denis Waast, Jean Armengaud, Ophelie Renoult, Claire Pecqueur, Yves Maugars, Marie-Astrid Boutet , Jerome Guicheux, Claire Vinatier
Cell Communication and signaling
Abstract and more

Gautier Tejedor,Prisca Boisguerin, Éric Vivès, Christian Jorgensen, Jérôme Guicheux, Claire Vinatier, Claire Gondeau, and Farida Djouad
Stem Cells International
 Transition from continuous to microglobular shaped peptide assemblies through a Liesegang-like enzyme-assisted mechanism.
Transition from continuous to microglobular shaped peptide assemblies through a Liesegang-like enzyme-assisted mechanism.Runser J-Y, Fneich F, Senger B, Weiss P, Jierry L, Schaaf P
Journal of Colloid and Interface Science, 2022 Nov 22
Abstract and more
 IL-34 deficiency impairs FOXP3+ Treg function in a model of autoimmune colitis and decreases immune tolerance homeostasis.
IL-34 deficiency impairs FOXP3+ Treg function in a model of autoimmune colitis and decreases immune tolerance homeostasis.Freuchet A, Salama A, Bézie S, Tesson L, Rémy S, Humeau R, Règue H, Sérazin C, Flippe L, Peterson P, Vimond N, Usal C, Ménoret S, Heslan JM, Duteille F, Blanchard F, Giral M, Colonna M, Anegon I, Guillonneau C.
Clin Transl Med. 2022 Aug;12(8):e988. doi: 10.1002/ctm2.988.
Abstract and more









PhD defense & HDR

Flyer de l'unité
Réunion club Devstem

22/04/2025; 10/06/2025
Voici le thème de cette année : Spotlight on Stem Cells: "Cell fate decision in vivo and in vitro"
Plus d'information à venir...
Le Club Macrophage Grand Ouest
Le club Macrophage Grand Ouest regroupe des équipes de recherche de Nantes, Angers, Rennes et Tours. Il a notamment pour but de favoriser les échanges scientifiques et techniques entre les équipes travaillant sur les macrophages et autres phagocytes mononucléés, d'aider les collaborations locales, et de donner l'opportunité aux jeunes de présenter leurs travaux de recherche.
l'inscription au Club est possible sur notre site: https://macrophage-grandouest.fr/
Le congrès "Resolution Days" qui se tiendra du 8 au 10 avril 2025 à Francfort
https://www.resolutiondays.co/